 Advanced Cell Technology’s (ACT) cell therapy programs are built upon a new class of cells able to form virtually every cell type in the body. This technology platform therefore has unusually broad applications in medicine. These primordial stem cells include Embryonic Stem (ES) cells and other cells from the Inner Cell Mass (ICM) of preimplantation embryos. The biotechnology industry hopes to produce many new therapies from these cells, for instance, neurons for the treatment of Parkinson’s disease and spinal cord injury, heart muscle cells for heart failure, cartilage for arthritis, pancreatic cells for diabetes, as well as many others.
Advanced Cell Technology’s (ACT) cell therapy programs are built upon a new class of cells able to form virtually every cell type in the body. This technology platform therefore has unusually broad applications in medicine. These primordial stem cells include Embryonic Stem (ES) cells and other cells from the Inner Cell Mass (ICM) of preimplantation embryos. The biotechnology industry hopes to produce many new therapies from these cells, for instance, neurons for the treatment of Parkinson’s disease and spinal cord injury, heart muscle cells for heart failure, cartilage for arthritis, pancreatic cells for diabetes, as well as many others.
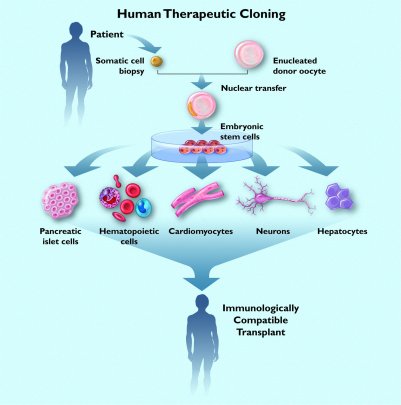
As promising as ES cell technology may seem, it does not solve the critical problem of histocompatibility. Human ES cells obtained from embryos derived during in vitro fertilization procedures, or from fetal sources, are essentially cells from another individual (allogeneic). This means that they, or any cells made from them, would be at risk of being rejected if transplanted into a human being. To solve this problem, ACT is performing research on three means to manufacture embryonic cells identical to a human adult, this is to say, autologous embryonic cells.